Acid Attack - Occurrence
A. Bacterial Environment
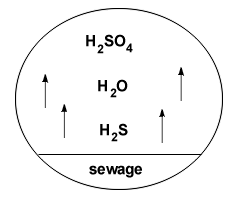
By far, the most common occurrence of acid attack are those found in sanitary sewers and waste water treatment facilities. In most cases, the acidic environment is produced by sulfur reducing bacteria. Some bacteria metabolize sulfates anaerobicaly and produce H2S as a waste product while others reduce sulfur and oxidize it back into H2SO4. The bacteria genus Thiobacillus is the most destructive to concrete, remaining active in concentrations as high as 5% H2SO4. (ACI 210R-93, 1996)
Key environmental criteria for these bacteria to produce H2S are a free water surface, low dissolved oxygen, and a low waste flow velocity. The illustration below depicts the most common deterioration scenario occurring in sewers.

H2S + H2O ![]() H2SO4
H2SO4
Hydrogen sulfide + water ![]() sulfuric acid
sulfuric acid
Acid attack deterioration following this scenario is extremely destructive. Concrete trunk sewers in warm climates may be stripped beyond reinforcement in as little as 10 years. Cases of asbestos coated sewer lines deteriorating in as little as 5 to 10 years are not uncommon. (ACI 210R-93, 1996)
A similar type of scenario involving sulfur reducing bacteria may be found in reservoirs and other natural water source containment structures. Although the mechanism of deterioration is similar the growth environment of the bacteria and hydrogen sulfide production are different.
Sulfate rich runoff caused by agricultural and or industrial activities collect in reservoirs providing the necessary "food" for the sulfate reducing bacteria. An anaerobic environment at the reservoir bottom caused by natural occurring thermalclines provides the necessary environment for the bacteria to metabolize the sulfate rich sediments thus producing hydrogen sulfide. This hydrogen sulfide will normally remain dissolved at the reservoir bottom unless disturbed by outside influence. This outside disturbance caused by water regulating siphons exposes the H2S rich waters to concrete outlets and sluice gates of the dam. The H2S rich waters, if allowed to reach low enough velocities will allow H2S build up and deterioration similar to that experienced in sewer systems will occur. (Thornton, 1978)
B. Carbonic acid attack (Aggressive CO2 Environment)
Acid attack of concrete can also be caused by exposure to water containing an excessive amount of carbonic acid also referred to as aggressive CO2 . Free CO2 dissolved in water takes the form of H2CO3 and if not in the presence of adequate amounts of calcium bicarbonate Ca(HCO3)2 will contribute to the deterioration of concrete. The destructive reaction of aggressive CO2 and concrete is a function of the ratio of H2CO3 and Ca(HCO3)2 to which the concrete is exposed. Both Ca(HCO3)2 and H2CO3 will react with Ca(OH)2 in concrete paste forming CaCO3, as illustrated in reactions 1 and 2 below. If the water, to which concrete is exposed, contains the minimum H2CO3 to maintain equilibrium with Ca(HCO3)2, calcium carbonate CaCO3 will form until the pores in the concrete are filled and no damage to concrete will occur. (Terzaghi, 1948)
CH + H2CO3 ![]() CaCO3 + 2H2O (1)
CaCO3 + 2H2O (1)
Calcium Hydroxide+ Carbonic acid ![]() Calcium Carbonate + water
Calcium Carbonate + water
CH + Ca(HCO3)2 ![]() 2CaCO3 + 2H2O (2)
2CaCO3 + 2H2O (2)
Calcium Hydroxide+ Calcium bicarbonate ![]() Calcium Carbonate+ water
Calcium Carbonate+ water
If, however, an excess amount of H2CO3 is present, the additional amount of H2CO3 will react with CaCO3 reforming Ca(HCO3)2 which is readily removed in solution. This process increases both the porosity and permeability of the cement paste.
Conditions under which this phenomena occurs are difficult to predict but the application of some simple tests will accurately identify the presents of an aggressive CO2 environment. Areas were volcanic activity is recent "geologically" are particularly susceptible due to the large amounts of dissolved CO2 in ground water caused by volcanic activity. Large amounts of decaying organic material coupled with pressure due to water depth is characteristic of river, marsh, and reservoir bottom environments and can also contribute to large amounts of dissolved CO2. The production of dissolved CO2 in water is also influenced by aquatic plant life growth which may be affected by the introduction of industrial waste. Under normal conditions, however, the percolation of ground waters through calcareous material, common to most subsurface conditions, will provide adequate amounts of Ca(HCO3)2 and thus decrease the effects of aggressive CO2. If however, the Ca(HCO3)2 to H2CO3 ratio is diluted as is the case in brackish coastal waterways, aggressive CO2 will be present in solution. (Terzaghi, 1978)